Director: Dr. Daohong Zhou
The HTS Facility is a state-of-the-art technological hub that performs HTS of chemical and natural product libraries.
The core is located on the third floor of the Barshop Institute for Longevity and Aging Studies (about 2,500 sq. ft.) at the UTHSA Greehey Research Campus. It is fully equipped with cell culture facilities and a full suite of detection, robotic and imaging equipment designed to perform work in microwell plates (96- or 384-well formats).
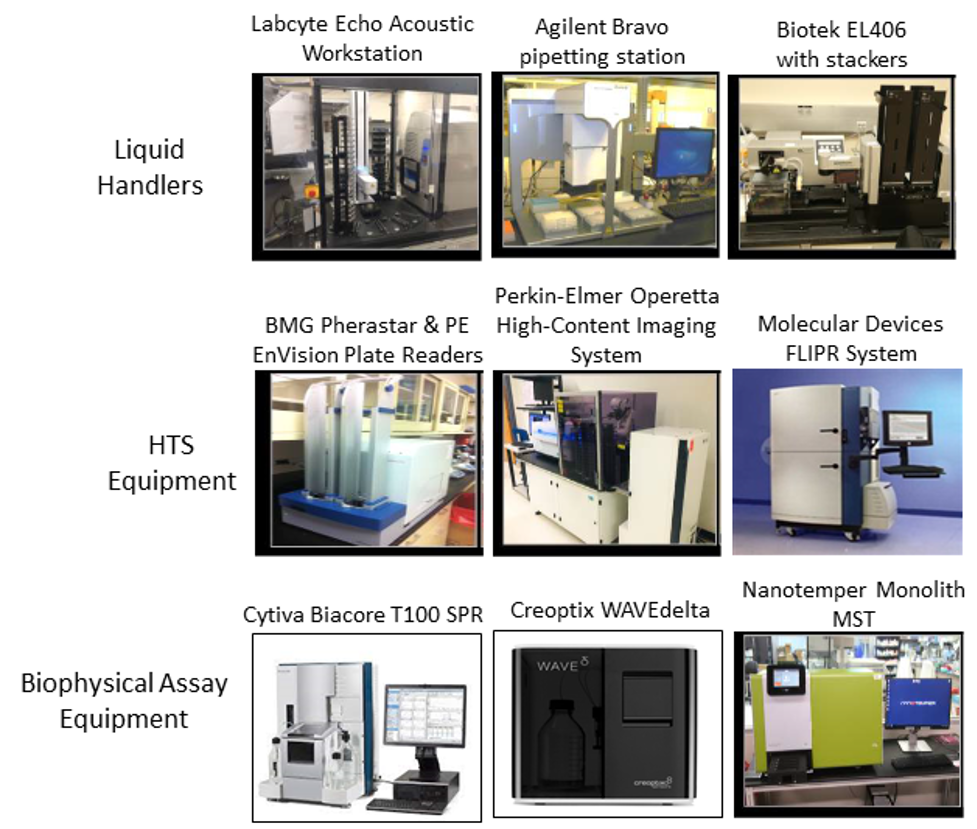
The Facility has liquid handling systems (two Agilent Bravo and Perkin Elmer Janus pipetting stations; BioTek EL406, and the Labcyte Echo Acoustic Dispenser) that perform, with high precision, the steps necessary for automated screens of its chemical libraries.
"State-of-the-art” Detection Instrumentation
- The Perkin Elmer Operetta cellular high-content imaging system: This system is a widefield and fluorescence microscope with confocal capability and excitation/emission filters to detect fluorophores ranging from UV to far-red in an environmentally-controlled chamber, robotically interfaced with a Liconic Cell incubator to allow for fully-automated live-cell experiments.
- The Molecular Devices FLIPR® Penta System is an HTS system for real-time kinetic cellular assays to monitor GPCRs and ion channels using both fluorescent and luminescent-based technologies. It is well suited to evaluating the toxicity of chemical compounds using organoids and stem cell-derived neuronal and cardiac cells.
- A Biacore T100 Surface Plasmon Resonance (SPR) instrument with T200 enhanced sensitivity from GE: SPR is a sensitive spectroscopic tool for studying molecular interactions in a label-free state. The system detects binding in real-time by measuring mass changes of a target immobilized on a chip upon analyte binding. It measures dissociation constant (KD), association rate constant (Kon or Ka), and dissociation rate constant (Koff or Kd) and thus provides kinetic parameters. SPR is broadly compatible the analysis of proteins, nucleic acids, lipids, and small molecules.
- The Creoptix® WAVEdelta system: The WAVEdelta system uses the Grating-Coupled Interferometry (GCI) technology to measure ligand binding and binding kinetics as SPR but provides higher throughput, sensitivity, and signal-to-noise ratio than SPR. As such, it is considered a next generation bioanalytical instrument for drug discovery and life science research.
- Nanotemper Monolith Microscale Thermophoresis NT: This is an automated system shared with the Greehey Children Cancer Research Institute Target Identification Facility, which can perform in-solution assays to measure binding affinities of protein-protein, protein-DNA/RNA or protein-drug interactions.
Featured Instrumentation: Creoptix® WAVEsystem
 The Creoptix® WAVEsystem combines the unrivaled high sensitivity of the GCI in the WAVEcore with the WAVEcontrol software to give you superior data analysis and the WAVEchips, our no-clog microfluidic cartridges, to support a wide range of sample types and sizes. The WAVEsystem comes with a temperature-controlled autosampler that fits vials, two 96- or one 384-well plates for further ease of use. In its entirety, the system gives you an unparalleled platform for real-time label-free binding kinetics.
The Creoptix® WAVEsystem combines the unrivaled high sensitivity of the GCI in the WAVEcore with the WAVEcontrol software to give you superior data analysis and the WAVEchips, our no-clog microfluidic cartridges, to support a wide range of sample types and sizes. The WAVEsystem comes with a temperature-controlled autosampler that fits vials, two 96- or one 384-well plates for further ease of use. In its entirety, the system gives you an unparalleled platform for real-time label-free binding kinetics.
- Crude samples, harsh chemicals, and particles up to 1000 nm
- Self-contained disposable cartridge and small footprint
- Ultra-fast transition times of 150 ms with reliable determination of off-rates up to 10 s-1
- Superior signal-to-noise ratios (0.01 pg/mm2 at 1 Hz)
- Reliable kinetics and binding affinities (KD) from low pM to low mM with from signals below 1 pg/mm2
- Analyze large ligand‑to‑analyte molecular weight ratios up to >1000:1
- Up to 120 hours of unattended operation
Researchers
The CIDD High Through-Put Screening Core Facility consists of full time, dedicated staff possessing an extensive amount of industry level experience from both the pharmaceutical and contract research areas.
Guiming Li, PhD- 7 years at CIDD
- Plate-based HTS
- Imaging-based HTS
Srikanth Polusani, PhD- 5 years at CIDD
- Plate-based HTS
- Imaging-based HTS
Lin Cao, MS- 4 years at CIDD
- Assists in various assays
Chemical Libraries
~171,000 cmpd sourced from ~3.0 million cmpd
- Maybridge HitFinder (14,400)
- Chembridge NovaCore (20,000)
- Chembridge DiverSet (30,000)
- Life Chemical Fsp3 (25,600)
- Life Chemical Diversity Set (56,000)
- UTSA Select (>2,500)
- Covalent Inhibitors (3508)
- Ion Channel Inhibitors (9000)
- Now sourcing: Macrocyclic compounds (complex, unique chemical space, targeting protein-protein interfaces)
- SIGMA LOPAC (1,280)
- Prestwick FDA-Approved (1,200)
- TargetMol Bioactives (4,400)
Initial screens using the latter libraries allow for repurposing of drugs with known clinical features for new therapeutics applications, enabling researchers to assign new IPs to these compounds. With a great deal of clinical data already available for these drugs, investigators can rapidly progress to preclinical animal models.
CIDD Vault
A powerful web-based database management and analysis system, the Collaborative Drug Discovery vault (CDD Vault), will track usage of chemical libraries and performs data analysis to identify lead candidates, including “on the fly” data analysis, enabling rapid identification of potential hits.
By using fluorescence-based readouts combined with the analysis of cell morphological properties, investigators can utilize the tools provided by the Harmony and Columbus software to perform powerful multiparametric studies of cell behavior upon treatment with chemical libraries or to analyze cell behavior in response to treatment with biological ligands or changes in growth conditions.
All small molecules are registered in the CDD vault, which provides tools to import screening datasets and analysis methods to query these data and perform diversity analyses of chemical libraries as well as searches by chemical substructure and/or chemical similarity and clustering algorithms. In addition, it provides investigators with standardized tools to view, analyze and then prioritize their screening data by expert chemists.
Contact Information
CIDD at UTHSA
Daohong Zhou: zhoud@uthscsa.edu
Guiming Li: lig@uthscsa.edu
Srikanth Polusani: polusani@uthscsa.edu
